i-Particles® and i-LipoP®-based products
Personalized formulations
Analytical services
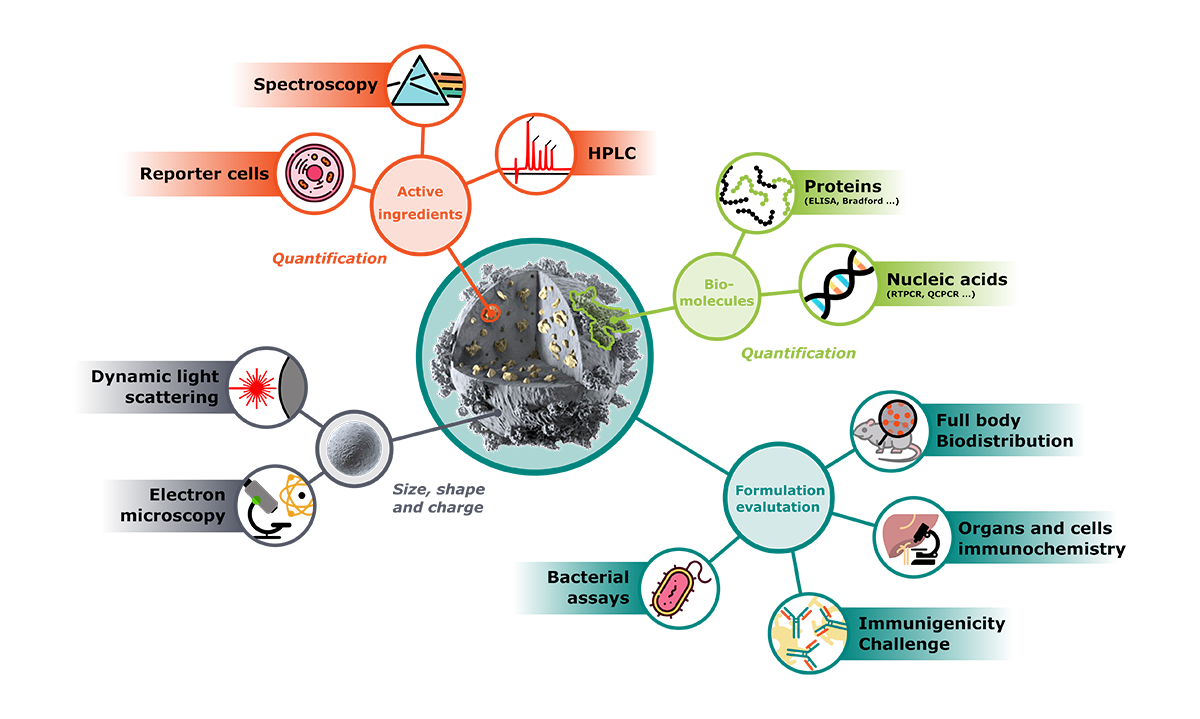
A complete analysis package
Morphological characterization
Active ingredients analysis
In vitro and in vivo evaluation
Find out our product catalog
Plain
i-Particles®
Work with the original

Size
From 100 to 500 nm
Buffer
Water – Saline – Phosphate – Carbonate – …
Active
i-Particles®
Potentiate active ingredients
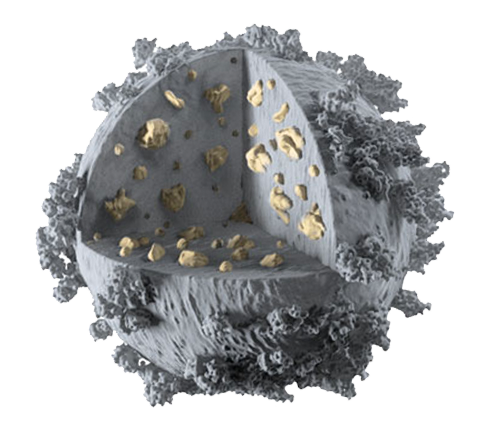
Encapsulation
Antibiotics – Antioxidants – TLR/NOD ligands – …
Adsorption
Peptides – Antigens – Enzymes – …
Fluorescent, dyed & contrasting
i-Particles®
Visualize your formulation

Fluorophores
360/425 nm – 505/515 nm – 600/620 nm
635/645 nm – 660/675 nm – 760/770 nm
Contrast agent
Gold – Iron oxide – Iodinated
Dye
Rhodamine 6B – Rhodamine G – …
Plain
i-LipoP®
Work with the original

Size
230 nm
Buffer
Saline
Active
i-LipoP®
Potentiate active ingredients
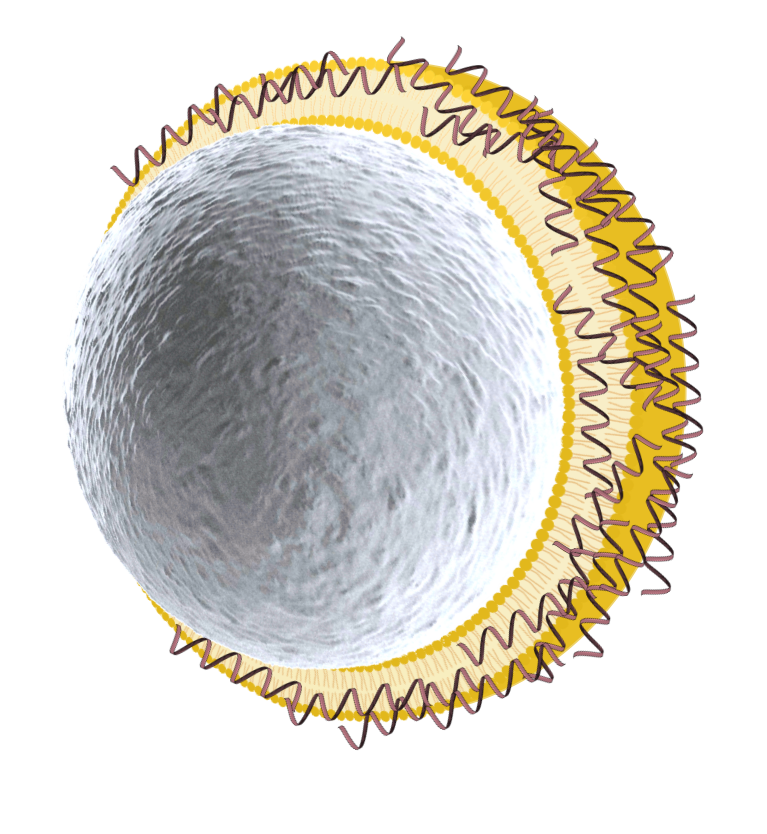
Adsorption
mRNA – dsRNA – DNA – …
Fluorescent
i-LipoP®
Visualize your formulation
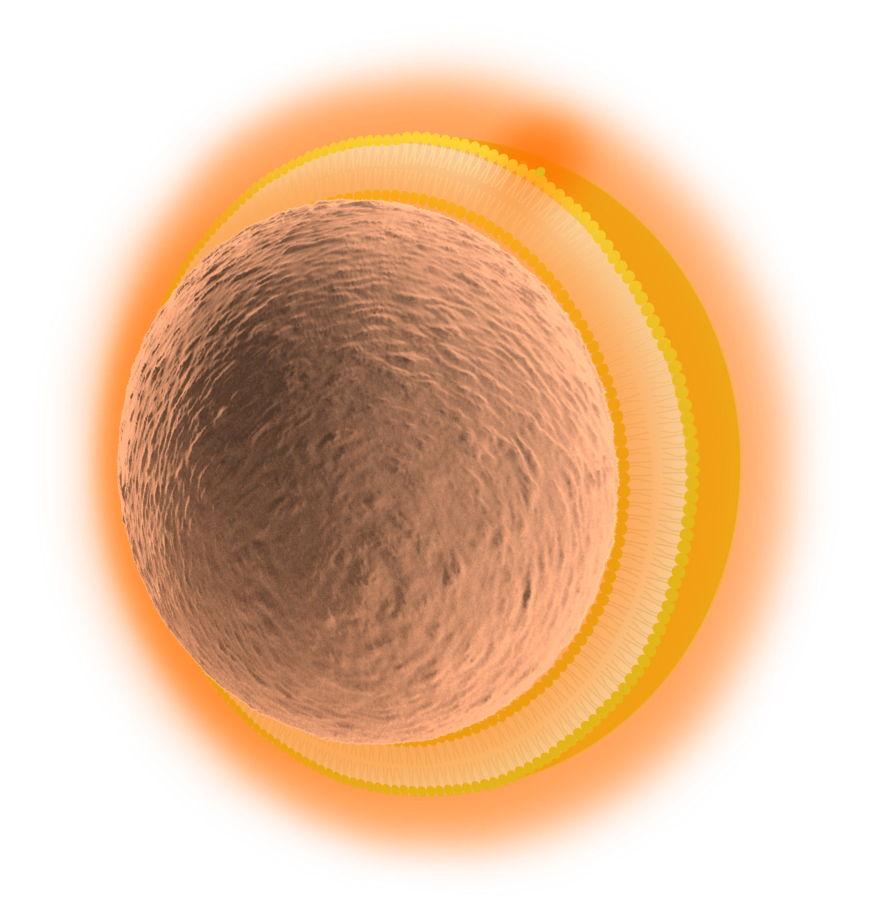
Fluorophores
600/620 nm – 760/770 nm
Research purposes only. Not intended for human or animal diagnostic, therapeutic or commercial use.
Develop your own formulation,
unlock your product full potential
From design to customer support, Adjuvatis is driven by quality to achieve customer expectations in all development steps. While i-Particles® are dedicated to hydrophobic molecule encapsulation and surface loading of proteins such as peptides, enzymes and antigens, i-LipoP® made possible the vectorization of nucleic acids such as mRNA.
Design
• Pharmaceutical data collection
• Loading prediction
• Specification definition
• Strategy proposition
Development
• Feasibility study
• Formulation optimization
• Medium/large scale production
• Analysis method
Characterization
• Colloidal and drug analysis
• Formulation stability
• In vitro and in vivo evaluation
• BSL2/3 subcontracting
Support
• Regular follow-up
• Data transfer
• Scientific expertise
• Full study report
An evaluation package for high quality analysis
Complying with quality standards, Adjuvatis is able to supply a complete characterization of i-Particles® and i-LipoP® formulations.
The analytical services include chemical formulation analysis, physico-chemical characterization and in vitro and in vivo evaluation.
Malvern Zetasizer Ultra Red
Adjuvatis proposes an analysis service based on its Zetasizer Ultra Red from Malvern Panalytical
[ess_grid alias= »images-lyon »]
